

FDA/510K CE David/OEM Service HCG Pregnancy Test
Basic Info.
| Model NO. | RH-HCG |
| Specification | 6.0mm |
| Trademark | DAVID, RUNBIO, OEM |
| Origin | Shantou, China |
| Production Capacity | 400000 Pieces Per Day |
Product Description
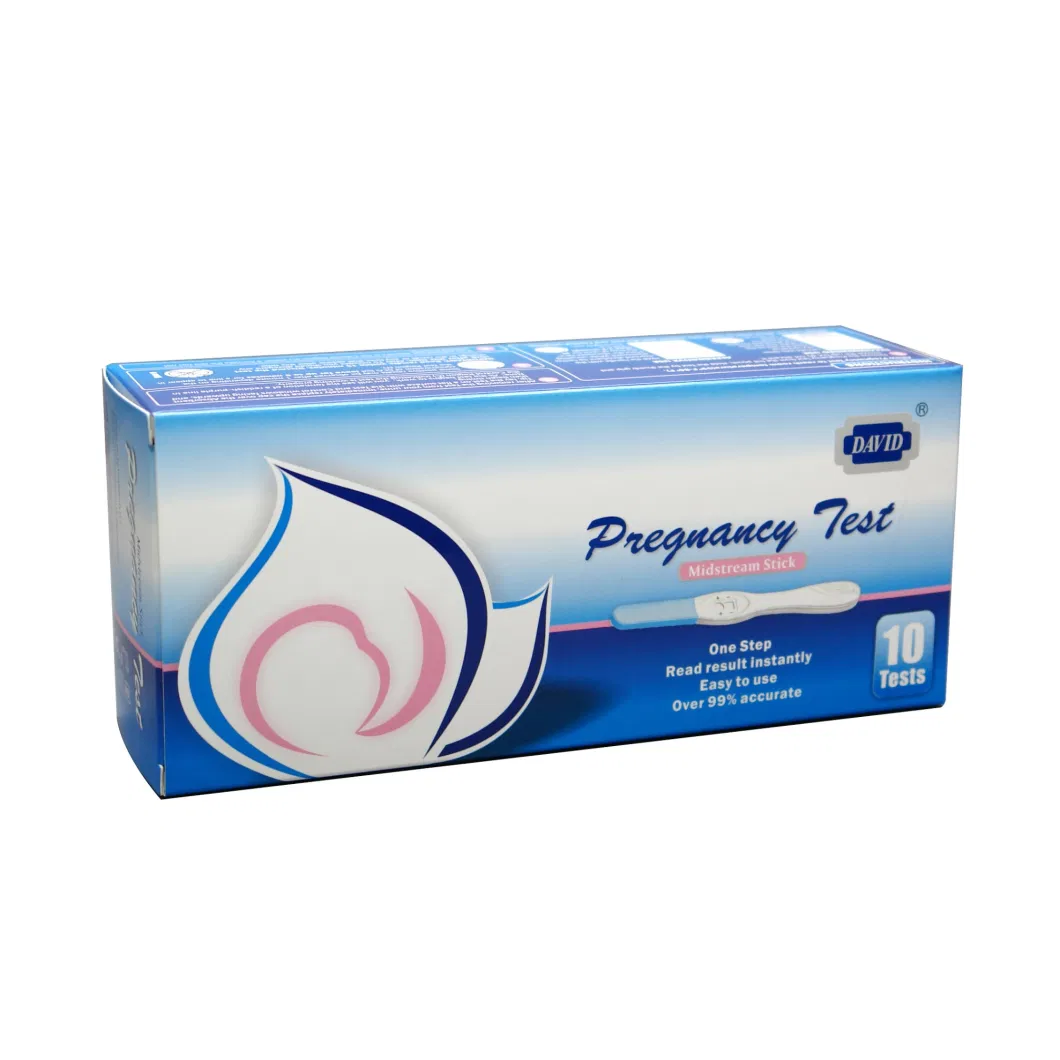
HCG Pregnancy Test| Product name | HCG Pregnancy test |
| Sensitivity | 10 miu/ml;15miu/ml;25miu/ml |
| Accuracy | more than 99% |
| Shelf time | 24-36 month |
| OEM time | 15-35 days |
| Certificate | CE/ISO13485/FDA |
| Specimen | Urine,Serum |
| Format | Strip /Cassette /Midstream |
| Size | Strip: 2.5mm/3.0mm/3.5mm/4.0mm/5mm |
| Cassette :3mm/4.2mm | |
| Midstream:4mm/6mm | |
| Usage | Read results within 10-15 minutes |

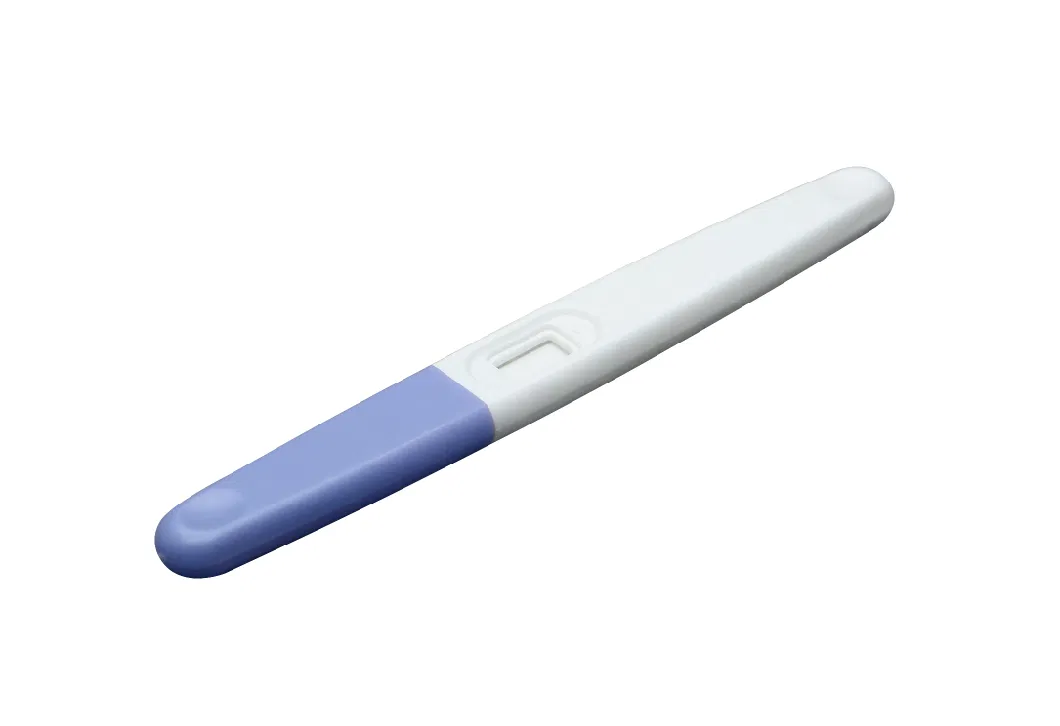

How to operate the test
1. When you are ready to begin testing, open the foil pouch by tearing along the notch. Remove the test and take off the cap.
2. With the test pointing downwards hold the absorbent sampler in your urine stream for 2-3 seconds (please do not stream pass the arrow end and taking care not to splash the window.)You may then wish to replace the cap. 3. Hold the test for a few seconds or lay the test flat. When you see the urine pass the show window and red line appear, the test is complete and has worked correctly. Read the result in 5 minutes but no longer than 15 minutes.
Note: Any urine specimen is appropriate for the HCG test midstream, but if you have just begun your pregnancy, the first morning urine is optimal.
How to read the results
*Negative: If there is only one red line in the show window, it means that you are not pregnant.
*Positive: If there are two red lines appear in the show window, it means that you are pregnant.
*Invalid: A total absence of red lines in the show window is an indication of procedure error and/or that test reagent deterioration has occurred.








